A few weeks ago, we published a 5-step guide on how to choose the right disinfectant in the market, in which we mentioned the efficacy and material compatibility of a disinfectant. This is largely dependent on the disinfectant’s active substance, the main component which gives the disinfectant its biocidal activity. Here, we compare the most common active substances found in chemical disinfectants and antiseptics, comparing their strengths and weaknesses to take you a step further in choosing the right product.
Alcohol (60-90%)
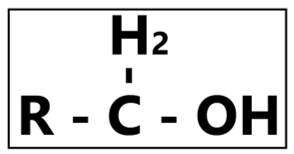 The common alcohols present in our disinfectant products are ethyl alcohol (ethanol), isopropyl alcohol (propanol) or sometimes a combination of both these substances. Alcohol’s kill approach would be their ability to denature proteins. Apart from its excellent bactericidal, fungicidal, tuberculocidal, and limited virucidal effects, alcohol is not recommended to be used to achieve sporicidal effects. The optimum alcohol concentration to be used is between 60% and 90%; concentrations of 50% and below have been shown to produce a lesser active response towards microorganisms. Selecting alcohol as an active substance brings the advantage of a fast acting, non-residual, and low cost production of a disinfectant product. Precautions are to be taken when utilizing alcohol as an active substance due to its flammable capabilities. It also hardens rubber and/or cracks acrylate plastic when in contact. Despite its many advantages, one must keep in mind that alcohols are not meant to be cleaning agents, and it is highly recommended to wash contaminated hands (with organic material) prior to disinfecting with an alcohol-based disinfectant for a more effective outcome.
The common alcohols present in our disinfectant products are ethyl alcohol (ethanol), isopropyl alcohol (propanol) or sometimes a combination of both these substances. Alcohol’s kill approach would be their ability to denature proteins. Apart from its excellent bactericidal, fungicidal, tuberculocidal, and limited virucidal effects, alcohol is not recommended to be used to achieve sporicidal effects. The optimum alcohol concentration to be used is between 60% and 90%; concentrations of 50% and below have been shown to produce a lesser active response towards microorganisms. Selecting alcohol as an active substance brings the advantage of a fast acting, non-residual, and low cost production of a disinfectant product. Precautions are to be taken when utilizing alcohol as an active substance due to its flammable capabilities. It also hardens rubber and/or cracks acrylate plastic when in contact. Despite its many advantages, one must keep in mind that alcohols are not meant to be cleaning agents, and it is highly recommended to wash contaminated hands (with organic material) prior to disinfecting with an alcohol-based disinfectant for a more effective outcome.
Quaternary Ammonium Compounds
The colorless, odorless, and highly stable quaternary ammonium compounds (QACs) are widely used as an active substance in an alcohol-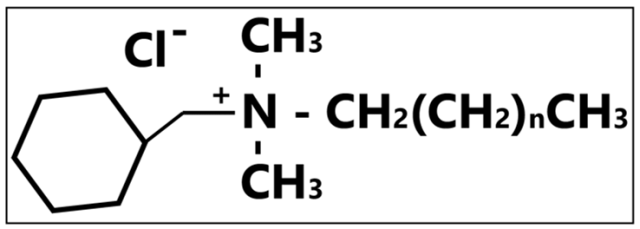 free disinfectant product. These compounds include alkyl dimethyl benzyl ammonium chloride, dodecyl dimethyl ammonium bromide and many more. QACs’ mode of action is described to be the inactivation of energy-producing enzymes, the denaturing of essential cell proteins, and the disruption of cellular membrane causing leakage of cellular constituents. QACs are not tuberculocidal and are inactive towards non-enveloped viruses. These compounds are best suited for bactericidal activity against Gram positive bacteria, fungicidal activity, and virucidal activity against enveloped viruses. QACs are also known to be deodorizing, with detergent action, however, despite the additional advantage, some QACs do not perform as effective when in contact with materials such as cotton or gauze pad. Aside from absorbing the active ingredients when in contact with cotton or gauze pads, some QACs-incorporated products may give rise to insoluble precipitation when in use.
free disinfectant product. These compounds include alkyl dimethyl benzyl ammonium chloride, dodecyl dimethyl ammonium bromide and many more. QACs’ mode of action is described to be the inactivation of energy-producing enzymes, the denaturing of essential cell proteins, and the disruption of cellular membrane causing leakage of cellular constituents. QACs are not tuberculocidal and are inactive towards non-enveloped viruses. These compounds are best suited for bactericidal activity against Gram positive bacteria, fungicidal activity, and virucidal activity against enveloped viruses. QACs are also known to be deodorizing, with detergent action, however, despite the additional advantage, some QACs do not perform as effective when in contact with materials such as cotton or gauze pad. Aside from absorbing the active ingredients when in contact with cotton or gauze pads, some QACs-incorporated products may give rise to insoluble precipitation when in use.
Peracetic Acid
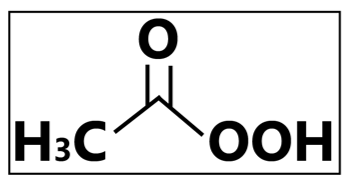 “The Environment Jackpot.”—Known to be quite environmentally friendly, peracetic acid produces acetic acid, water, oxygen, and hydrogen peroxide when decomposed into the environment. Safe and effective, this active substance is highly sporicidal, bactericidal, yeasticidal, fungicidal, virucidal, mycobactericidal, and tuberculocidal. Peracetic acid inactivates microorganism by cellular protein denaturation, disruption of cell membrane, and oxidizing the sulphur bonds in cellular metabolites. This compound also demonstrates good biocidal effects in the presence of organic materials. Although its advantages are quite attractive, peracetic acid has known to have a higher operation and purchase cost. Moreover, it is only suitable for immersion sterilization , causes eye and skin damage when in contact with concentrated products, and are corrosive towards some metals. Proper handling and consideration is needed when using peracetic acid products.
“The Environment Jackpot.”—Known to be quite environmentally friendly, peracetic acid produces acetic acid, water, oxygen, and hydrogen peroxide when decomposed into the environment. Safe and effective, this active substance is highly sporicidal, bactericidal, yeasticidal, fungicidal, virucidal, mycobactericidal, and tuberculocidal. Peracetic acid inactivates microorganism by cellular protein denaturation, disruption of cell membrane, and oxidizing the sulphur bonds in cellular metabolites. This compound also demonstrates good biocidal effects in the presence of organic materials. Although its advantages are quite attractive, peracetic acid has known to have a higher operation and purchase cost. Moreover, it is only suitable for immersion sterilization , causes eye and skin damage when in contact with concentrated products, and are corrosive towards some metals. Proper handling and consideration is needed when using peracetic acid products.
Hydrogen Peroxide
Hydrogen peroxide is widely used in the disinfectant manufacturing industry. Inclusive of high-level disinfectant efficacy against bacterial spores, Hydrogen peroxide is also bactericidal, fungicidal, virucidal, and mycobactericidal effective. Hydrogen peroxides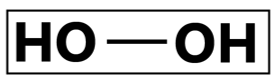 inhibit microorganism cellular activity by producing easy-pass through hydroxyl free radicals that disrupts microorganism membrane lipids causing a leakage of cellular components. This oxidative biocidal compound is also known to cause cellular protein synthesis disruption. Hydrogen peroxide has been positively regarded due to its effectiveness and stability. This compound is odorless, does not leave residues, removes organic materials present on fabrics and are generally environmentally safe due to its ability to degrade into oxygen and water. However, despite its positive characteristics, the material compatibility issue and irritation effect of Hydrogen peroxide has been raised, this compound has been reported to cause ocular irritation when exposed in the eye. Proper handling and care should be taken when working with brass, copper or items or equipment with silver plating in the presence of Hydrogen peroxide as it may result in corrosion effect.
inhibit microorganism cellular activity by producing easy-pass through hydroxyl free radicals that disrupts microorganism membrane lipids causing a leakage of cellular components. This oxidative biocidal compound is also known to cause cellular protein synthesis disruption. Hydrogen peroxide has been positively regarded due to its effectiveness and stability. This compound is odorless, does not leave residues, removes organic materials present on fabrics and are generally environmentally safe due to its ability to degrade into oxygen and water. However, despite its positive characteristics, the material compatibility issue and irritation effect of Hydrogen peroxide has been raised, this compound has been reported to cause ocular irritation when exposed in the eye. Proper handling and care should be taken when working with brass, copper or items or equipment with silver plating in the presence of Hydrogen peroxide as it may result in corrosion effect.
Chlorine Compounds
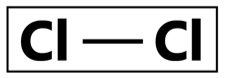 “Isn’t chlorine bleach?” or “Chlorine as in swimming pools?” —Well, yes and yes. Sodium hypochlorite is the most widely used liquid chlorine disinfectant. With a broad spectrum of activity, chlorine compounds are known to be bactericidal, yeasticidal, fungicidal, virucidal, tuberculocidal, and sporicidal. The killing action of these compounds includes the inhibition of cellular protein synthesis, decrease in DNA synthesis, an increase in DNA breakage, decrease in cellular oxygen uptake, and chlorination of amino acids amongst others. Apart from being a fast acting compound, chlorine compounds do not leave residues, remove surface biofilms, and are of low cost. No doubt these advantages are attractive, but one should consider the potential of chlorine compounds being hazardous, producing side effects including eye irritation and burns in the gastric channels. Additionally, the efficacy of chlorine compounds are known to decrease when in contact with organic materials. These compounds are highly not recommended for use with metal and rubber surfaces as it may cause damage and/or corrosion.
“Isn’t chlorine bleach?” or “Chlorine as in swimming pools?” —Well, yes and yes. Sodium hypochlorite is the most widely used liquid chlorine disinfectant. With a broad spectrum of activity, chlorine compounds are known to be bactericidal, yeasticidal, fungicidal, virucidal, tuberculocidal, and sporicidal. The killing action of these compounds includes the inhibition of cellular protein synthesis, decrease in DNA synthesis, an increase in DNA breakage, decrease in cellular oxygen uptake, and chlorination of amino acids amongst others. Apart from being a fast acting compound, chlorine compounds do not leave residues, remove surface biofilms, and are of low cost. No doubt these advantages are attractive, but one should consider the potential of chlorine compounds being hazardous, producing side effects including eye irritation and burns in the gastric channels. Additionally, the efficacy of chlorine compounds are known to decrease when in contact with organic materials. These compounds are highly not recommended for use with metal and rubber surfaces as it may cause damage and/or corrosion.
Glutaraldehyde
“The active substance that ages?”—Generally, these saturated dialdehydes, or commonly referred to as glutaraldehyde, are widely present in high-level disinfectant products. Glutaraldehyde exists in an acidic and alkaline form and when present at a pH 7.5 to 8.5,  is active against bacterial spores. When in alkaline form, the activated form has a shelf-life of minimum 14 days. However, this has been well improved with existing glutaraldehyde formulations such as glutaraldehyde-phenol-sodium phenate. These new formulations overcome the claims of which glutaraldehyde efficacy decreases with age with lasting shelf-life of up to 30 days when in use. However, a minimum range of 1.0% to 1.5% of glutaraldehyde concentration is recommended for an effective disinfection result. Other than being effective against bacterial spores, this compound is known to be bactericidal, fungicidal, virucidal, and mycobactericidal. Glutaraldehyde causes the alkylation of amino groups, hydroxyl, carboxyl, and many others in microorganisms leading to the alteration of cellular RNA, DNA, and protein synthesis leading to cellular inactivation. Disinfectant products with glutaraldehyde as the active substance are welcomed openly into hospitals and other healthcare settings due to its non-staining properties, non-corrosive with rubber and plastic equipment, and its capability to maintain biocidal effects when in contact with organic materials. With every good quality, the few considerations of this active substance are not to be taken lightly. Glutaraldehyde is best to be controlled and handle properly as the compound vapour, described to be pungent, has the potential of causing respiratory irritation and skin sensitization when exposed into the air. Proper handling and care is needed when in contact with exposed glutaraldehyde in poorly ventilated rooms or when used in open immersion baths for instruments.
is active against bacterial spores. When in alkaline form, the activated form has a shelf-life of minimum 14 days. However, this has been well improved with existing glutaraldehyde formulations such as glutaraldehyde-phenol-sodium phenate. These new formulations overcome the claims of which glutaraldehyde efficacy decreases with age with lasting shelf-life of up to 30 days when in use. However, a minimum range of 1.0% to 1.5% of glutaraldehyde concentration is recommended for an effective disinfection result. Other than being effective against bacterial spores, this compound is known to be bactericidal, fungicidal, virucidal, and mycobactericidal. Glutaraldehyde causes the alkylation of amino groups, hydroxyl, carboxyl, and many others in microorganisms leading to the alteration of cellular RNA, DNA, and protein synthesis leading to cellular inactivation. Disinfectant products with glutaraldehyde as the active substance are welcomed openly into hospitals and other healthcare settings due to its non-staining properties, non-corrosive with rubber and plastic equipment, and its capability to maintain biocidal effects when in contact with organic materials. With every good quality, the few considerations of this active substance are not to be taken lightly. Glutaraldehyde is best to be controlled and handle properly as the compound vapour, described to be pungent, has the potential of causing respiratory irritation and skin sensitization when exposed into the air. Proper handling and care is needed when in contact with exposed glutaraldehyde in poorly ventilated rooms or when used in open immersion baths for instruments.
Iodophors
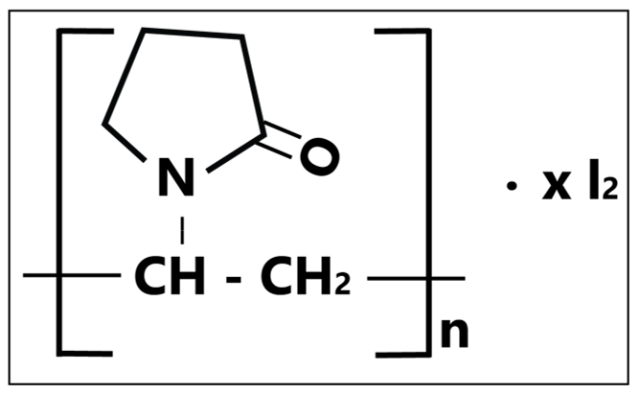 “Double Action Power.”—A combination of iodine and non-ionic detergents, iodophors act as an antiseptic and surface disinfectants. Iodophors are more desirable compared to iodine as they do not stain, are odourless, and are generally toxic- and irritant-free. The most commonly formulated iodophor compound is povidone-iodine. In addition to being effective under heavy soiled conditions, iodophor compounds are bactericidal, fungicidal, virucidal, mycobactericidal, and tuberculocidal. These compounds have shown limited sporicidal activity when exposed with a long contact time, though a prolonged contact time is not ideal especially when in need of fast disinfection. Of course, with many advantages, one should be informed of its inability to stay active under alkaline conditions and cause mild corrosion on some surfaces.
“Double Action Power.”—A combination of iodine and non-ionic detergents, iodophors act as an antiseptic and surface disinfectants. Iodophors are more desirable compared to iodine as they do not stain, are odourless, and are generally toxic- and irritant-free. The most commonly formulated iodophor compound is povidone-iodine. In addition to being effective under heavy soiled conditions, iodophor compounds are bactericidal, fungicidal, virucidal, mycobactericidal, and tuberculocidal. These compounds have shown limited sporicidal activity when exposed with a long contact time, though a prolonged contact time is not ideal especially when in need of fast disinfection. Of course, with many advantages, one should be informed of its inability to stay active under alkaline conditions and cause mild corrosion on some surfaces.
Phenolic Compounds
“The OG Disinfectant.”—Phenol were one of the first used compounds in hospitals. Various improved formulations of phenolic compounds have been derived, namely ortho-phenyl phenol and ortho-benzyl-para-chlorophenol, with improved biocidal spectrum that includes bactericidal, fungicidal, virucidal, and tuberculocidal. These compounds act through cellular protein precipitation and cellular membrane disruption, causing metabolic leakage. Despite its advantage of retaining its effectiveness in the presence of organic material, phenolic disinfectants should be handled with proper care in the nursery healthcare settings especially when exposed to a newborn. This is due to a study reported in the Centre of Disease Control and Prevention publications, presenting that cleaning incubators and infant bassinets with phenol-containing detergents or disinfectants prior to being occupied in, had resulted in an increase in infant bilirubin levels.
been derived, namely ortho-phenyl phenol and ortho-benzyl-para-chlorophenol, with improved biocidal spectrum that includes bactericidal, fungicidal, virucidal, and tuberculocidal. These compounds act through cellular protein precipitation and cellular membrane disruption, causing metabolic leakage. Despite its advantage of retaining its effectiveness in the presence of organic material, phenolic disinfectants should be handled with proper care in the nursery healthcare settings especially when exposed to a newborn. This is due to a study reported in the Centre of Disease Control and Prevention publications, presenting that cleaning incubators and infant bassinets with phenol-containing detergents or disinfectants prior to being occupied in, had resulted in an increase in infant bilirubin levels.
This concludes the mighty 8 active substances commonly used in the disinfectant manufacturing sector. What would be your choice of active substance? Let us know here and proceed with a testing journey in TECOLAB with our experts where we not only provide service, but solutions for our customers.
Reference:
1. Centers for Disease Control and Prevention. (2016). Guideline for Disinfection and Sterilization in Healthcare Facilities (2008). Retrieved from https://www.cdc.gov/infectioncontrol/guidelines/disinfection/disinfection-methods/chemical.html
2. Johansson, I., & Somasundaran, P. (2007). Handbook for Cleaning/Decontamination of Surfaces. Elsevier.

