Have you heard about the latest hot goss in the disinfectant industry? The Health Sciences Authority (HSA) of the Singapore government has announced a product recall of 18 hand sanitizer brands off the Singaporean market in February 2021. These products were recalled due to the detection of methanol and acetaldehyde that were not in compliance of the pharmaceutical pharmacopoeia limit. Pharmacopoeia refers to the quality specifications prepared by a national or regional authority of a specific country to control the quality and safety of a pharmaceutical product such as hand rubs.
So, What Are Methanol and Acetaldehyde? Why Should They be Avoided During Your Product Development and Manufacturing?
Amongst the many types of alcohols, ethanol and isopropanol (2-propanol) are most recommended to be used for alcohol-based hand rub (ABHR) by the World’s Health Organization (WHO), with an effective range between 60% and 80%. This recommendation could have sprouted from the fact that it is safer for human contact compared to methanol. Methanol, or commonly referred to as wood alcohol, is known to be at least 5 times cheaper than ethanol or isopropanol, perhaps making it more favorable to save cost. Unfortunately, a good deal does not necessarily guarantee safety.
Methanol
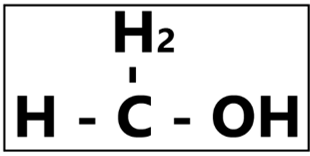 “The simplest form of alcohol.” – Did you know that methanol is used to make rocket fuel and windshield washer fluid? It is highly urged by the Food and Drug Administration (FDA) that this substance should never be applied onto our skin nor ingested. Methanol is a common additive in denatured ethanol production. Mostly due to cost, denatured alcohol is also commonly used as rubbing alcohol. Thus, the misuse of unapproved ethanol formulation with high methanol concentration in alcohol-based hand rubs leads to an undesirable exposure to the human body via inhalation, skin contact, or even accidental ingestion. Our body naturally breaks down methanol into formic acid, and an accumulation of this substance will lead to various undesirable effect such as nausea, vomiting, blindness, and more. Formic acid is also known to be a leading cause in nervous system damage that could eventually be lethal.
“The simplest form of alcohol.” – Did you know that methanol is used to make rocket fuel and windshield washer fluid? It is highly urged by the Food and Drug Administration (FDA) that this substance should never be applied onto our skin nor ingested. Methanol is a common additive in denatured ethanol production. Mostly due to cost, denatured alcohol is also commonly used as rubbing alcohol. Thus, the misuse of unapproved ethanol formulation with high methanol concentration in alcohol-based hand rubs leads to an undesirable exposure to the human body via inhalation, skin contact, or even accidental ingestion. Our body naturally breaks down methanol into formic acid, and an accumulation of this substance will lead to various undesirable effect such as nausea, vomiting, blindness, and more. Formic acid is also known to be a leading cause in nervous system damage that could eventually be lethal.
Acetaldehyde
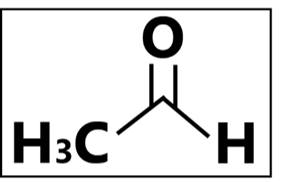 “Say no to carcinogens.” – In healthcare settings and food industries, pharmaceutical- and food-grade ethanol are generally much recommended. The COVID-19 pandemic has led to a shortage of pharmaceutical-grade alcohol, forcing manufacturers to use technical grade alcohols to produce hand sanitizers. Acetaldehyde is produced via the fermentation of ethanol production and it is also an impurity from the manufacturing environment such as the equipment and/or containers used. Contaminated ethanol is produced when acetaldehyde is inadvertently deposited into the end product. Being more easily evaporable than ethanol, the most common entry of acetaldehyde in humans would be via inhalation, followed by accidental ingestion, and skin contact. Acetaldehyde has also been proven to be carcinogenic, having the potential to cause cancer. An accumulation of acetaldehyde in the body causes skin, eye, and respiratory tract irritation as well as the physiological depression of the central nervous system.
“Say no to carcinogens.” – In healthcare settings and food industries, pharmaceutical- and food-grade ethanol are generally much recommended. The COVID-19 pandemic has led to a shortage of pharmaceutical-grade alcohol, forcing manufacturers to use technical grade alcohols to produce hand sanitizers. Acetaldehyde is produced via the fermentation of ethanol production and it is also an impurity from the manufacturing environment such as the equipment and/or containers used. Contaminated ethanol is produced when acetaldehyde is inadvertently deposited into the end product. Being more easily evaporable than ethanol, the most common entry of acetaldehyde in humans would be via inhalation, followed by accidental ingestion, and skin contact. Acetaldehyde has also been proven to be carcinogenic, having the potential to cause cancer. An accumulation of acetaldehyde in the body causes skin, eye, and respiratory tract irritation as well as the physiological depression of the central nervous system.
Benzene
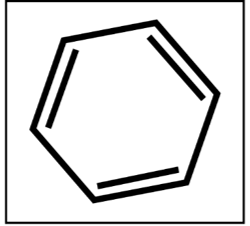 “Don’t judge a book by its cover.” Benzene, a sweet smelling yet highly flammable compound, is commonly used in the production of plastics, rubbers, and synthetic fibers. Benzene is a common additive added into the purification of ethanol in which water is removed from ethanol to produce pure ethanol in a process called dehydration through azeotropic distillation. With the use of benzene-contaminated alcohol in hand sanitizers, consumers are exposed to this substance via inhalation, skin absorption, and accidental ingestion. An accumulation of benzene in the body affects the bone marrow function, causing the reduce in the production of red blood cells and white blood cells. The latter are the soldiers of our body—compromising their production adversely affects our immune system. A long-term exposure to this cancer-causing substance also leads to leukemia.
“Don’t judge a book by its cover.” Benzene, a sweet smelling yet highly flammable compound, is commonly used in the production of plastics, rubbers, and synthetic fibers. Benzene is a common additive added into the purification of ethanol in which water is removed from ethanol to produce pure ethanol in a process called dehydration through azeotropic distillation. With the use of benzene-contaminated alcohol in hand sanitizers, consumers are exposed to this substance via inhalation, skin absorption, and accidental ingestion. An accumulation of benzene in the body affects the bone marrow function, causing the reduce in the production of red blood cells and white blood cells. The latter are the soldiers of our body—compromising their production adversely affects our immune system. A long-term exposure to this cancer-causing substance also leads to leukemia.
We’re Here to Help You Take Action Before You Get a Reaction
We’re here to help our customers screen their products for these harmful substances. The FDA has encouraged manufacturers to screen their disinfectants via gas chromatography and more to control the amount of these harmful substance at an acceptable limit (Table 1). TECOLAB is able to test if your alcohol-based product contains harmful impurities such as methanol, acetaldehyde, and benzene.
| Alcohol-Based Hand Sanitizer Screening | ||
| IMPURITIES | ACCEPTANCE LIMIT (PPM*) | RECOMMENDED ANALYSIS |
| Methanol | ≤ 630 | FTIR analysis (>3%) GC analysis (<3%) |
| Acetaldehyde | ≤ 50 | GC analysis |
| Benzene | ≤ 2 | GC analysis |
| * The acceptance limit stated are from the FDA guidance for the manufacturing of alcohol for alcohol-based hand sanitizers. 10,000 ppm (parts per million) equals 1% | ||
Do let us know your sample of interest and outcome desired and we will assist you with the best analysis method for your product. TECOLAB is also able to provide chemical analysis to determine the active concentration of ethanol, isopropanol, quaternary ammonium compounds, phenoxyethanol, and more. We highly recommend this analysis to ensure the stability or the shelf-life of your product. Stay tuned for our next blog discussing the requirements of stability testing for disinfectants!
Reference:
1. Alberta Health Services. (2020). Hand Sanitizer Made with Technical-Grade Ethanol Safety Consideration. Retrieved from https://www.albertahealthservices.ca/assets/info/ppih/if-ppih-covid-19-discussion-document-teg-hand-sanitizer.pdf
2. Bethany Hansen. (2020). Methanol Toxicity. Retrieved from https://toxedfoundation.org/methanol-toxicity/
3. Centers for Disease Control and Prevention. (2018). Facts about Benzene. Retrieved from https://emergency.cdc.gov/agent/benzene/basics/facts.asp
4. Centers for Disease Control and Prevention, The National Institute for Occupational Safety and Health (NIOSH). (2019). Benzene. Retrieved from https://www.cdc.gov/niosh/npg/npgd0049.html
5. Centers for Disease Control and Prevention, The National Institute for Occupational Safety and Health (NIOSH). (2011). METHANOL: Systemic Agent. Retrieved from https://www.cdc.gov/niosh/ershdb/emergencyresponsecard_29750029.html
6. Centers for Disease Control and Prevention, The National Institute for Occupational Safety and Health (NIOSH). (2019). Acetaldehyde. Retrieved from https://www.cdc.gov/niosh/npg/npgd0001.html
7. Food and Drug Adminstration, Center for Drug Evaluation and Research (CDER). (2021). Temporary Policy for Manufacture of Alcohol for Incorporation into Alcohol-Based Hand Sanitizer Products during the Public Health Emergency (COVID-19) Guidance for Industry. Retrieved from https://www.fda.gov/media/136390/download
8. Food and Drug Administration. Is Your Hand Sanitizer on FDA’s List of Products You Should Not Use?. Retrieved from https://www.fda.gov/consumers/consumer-updates/your-hand-sanitizer-fdas-list-products-you-should-not-use
9. Government of Canada. (2021). Technical-grade ethanol for the manufacture of hand sanitizers and hard-surface disinfectants during the COVID-19 pandemic: Risk assessment summary report. Retrieved from https://www.canada.ca/en/health-canada/services/drugs-health-products/natural-non-prescription/legislation-guidelines/covid19-technical-grade-ethanol-hand-sanitizer/risk-assessment-summary-report.html
10. Kosaric, N., Duvnjak, Z., Farkas, A., Sahm, H., Bringer-Meyer, S., Goebel, O., & Mayer, D. (2011). Ethanol. Ullmann’s Encyclopedia of Industrial Chemistry, 1–72.
11. World Health Organization & World Health Organization. (2009). WHO Guidelines on Hand Hygiene in Health Care. World Health Organization, Patient Safety.

