“Hey, Joanne! NPRA has recently announced a voluntary registration for surface disinfectants in Malaysia starting from 10th September last year. Let’s register our product!”—The recent surge of surface disinfectant demand and placement in the current Malaysian market has alerted the Ministry of Health (MOH) to regulate these products. Ever since then, the MOH has appointed the National Pharmaceutical Regulatory Authority (NPRA) as the official authorized regulatory agency to regulate surface disinfectants in Malaysia. Check out the table below extracted from NPRA to know more about the regulatory bodies or agencies in charge of specific product regulation.
| CATEGORY | USAGE | PRODUCT CLASSIFICATION | AUTHORIZED BODY/AGENCY REGULATORS |
| First | Used for sanitization for disinfection of skin/human and animal body parts (sanitizer, disinfectant, antiseptic) | I) Generic product (Non-scheduled poison/OTC)
– Topical antiseptic or disinfectant for human and animal skin for a medical purpose – Hand sanitizer, disinfectant, or surgical rub which are used by healthcare professionals for treatment procedures |
NPRA |
| II) Cosmetic
– Hand sanitizer for general hand hygiene without therapeutic claims |
NPRA | ||
| III) Medical device
– Alcohol swab/wipe applied to the skin prior to injection for medical purpose |
Medical Device Authority (MDA) | ||
| Second | Used for sanitization or disinfection of medical devices | Medical device | MDA |
| Third | Used for sanitization or disinfection of all types of surfaces (except on human, animal, or medical devices) | I) Surface disinfectant for non-porous surface | NPRA |
| II) Surface disinfectant for other types of surface is classified as general consumer product | No specific regulatory authority |
What are the Efficacy Tests I Need to Do Prior to Registering My Surface Disinfectant in Malaysia?
“I have done my efficacy testing according to the European Standards. Will NPRA accept my test reports?”—The answer is yes. Surface disinfectant efficacy claims are mandatory to be supported by efficacy test report proving its antimicrobial effectiveness. In the NPRA guidelines for surface disinfectant registration, partial guidelines of EN 14885 has been incorporated. Other efficacy testing standards of those by the American Society for Testing and Materials (ASTM), Association of Official Analytical Collaboration International (AOAC International) and other established equivalent standards are also acceptable by the NPRA. Not sure if the test standard you’re looking into is suitable for NPRA product registration? Let us know here and we can assist you further. Tabulated below are the minimum requirements of the European Standard given by NPRA to fulfill prior to surface disinfectant registration in Malaysia.
| PRODUCT TYPE | APPLICATION AREA | MINIMUM SPECTRUM OF ACTIVITY | MINIMUM REQUIREMENT FOR METHOD USED | MINIMUM TEST METHOD REQUIRED |
| Hard surface disinfectant | Healthcare institution | Bactericidal & Yeasticidal | Carrier Lab Test (Phase 2, Step 2)* | EN 13697 (without mechanical action) or EN 16615 (with mechanical action) |
| Virucidal | Virucidal (Phase 2, Step 1) | EN 14476 | ||
| Veterinary | Bactericidal & Yeasticidal | Suspension Lab Test (Phase 2, Step 1) | EN 1656 & EN 1657 | |
| Related to food preparation except hospital use | Bactericidal & Yeasticidal | Suspension Lab Test (Phase 2, Step 1) | EN 1276 & EN 1650 | |
| Domestic | ||||
| Institution | ||||
| *Written as ‘suspension lab test (Phase 2, Step 2)’ in the NPRA guideline. | ||||
How Does the Minimum Requirement of Efficacy Testing in Malaysia Differ from EN 14885?
Product Claiming Procedure
EN 14885 is a universally known European Norm that highlights specific European Standards according to specific product categories including non-porous and porous surface disinfectants. EN 14885 also describes these minimum requirement efficacy tests throughout the medical area, veterinary area and the food, industrial, domestic and institutional areas. These minimum requirements are set according to 3 phases summarized in the table below.
| PHASE | TYPE OF TEST | INTERFERING SUBSTANCE | USED FOR PRODUCT CLAIM | EXAMPLE |
| Phase 1 | Suspension test | No | No | EN 1040, EN 1275 & EN 14347 |
| Phase 2, Step 1 | Suspension test under simulated practical conditions | Yes | Yes | EN 13727, EN 13624, EN 14476 etc. |
| Phase 2, Step 2 | Laboratory test under simulated practical conditions | Yes | Yes | EN 14561, EN 13697, EN 16615 etc. |
| Phase 3 | Field test | – | – | Under development |
According to the guidelines in EN 14885, Phase 2, Step 1 and Phase 2, Step 2 are mandatory to be done in order to claim for product efficacy activity. Phase 1 tests are not a mandated requirement for efficacy testing due to the lack of interfering substances that do not mimic real-life application exposure as compared to Phase 2 tests. The phase requirement has only been partially implemented in the current NPRA guidelines. Below is an illustrated guide for the minimum requirements of non-porous hard surface disinfectant claims in the provisional EN 14885:2020.
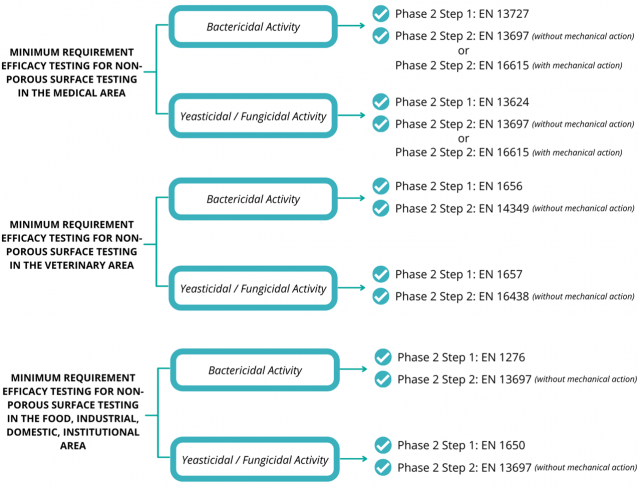
Differences between the NPRA Guideline and EN 14885
“Which guideline should I follow?”—The NPRA guideline was developed in reference to EN 14885. The NPRA guideline only specifies tests required for product registration in Malaysia. Our advice to you would be to plan out your efficacy testing with considering the countries or continent you would wish register your products in. If you are planning to register your product in Malaysia and in countries of the European Union, it would be better to implement the guidelines of EN 14885 in order to fulfill one of the few requirements of product registration required in the European Union, keeping in mind that EN 14885 envelopes the requirements of the NPRA. Here are the main differences between the two guidelines:
| DIFFERENCES | EN 14885 | NPRA GUIDELINES |
| Minimum requirements | All three focus areas of the standard include Phase 2, Step 1 and Phase 2, Step 2 tests as a minimum requirement | The requirements of Phase 2, Step 1 and Phase 2, Step 2 tests are inconsistent amongst the three focus areas of the guideline |
| Virucidal activity claims | Not a minimum requirement in the medical area | A minimum requirement in the medical area |
| Real-life application scenarios | Phase 2, Step 2 testing are widely available in all three focus areas to mimic real-life application under simulated conditions, e.g., the use of vinyl surfaces or metal discs that mimics facility surfaces | Real-life application of Phase 2, Step 2 testing are only available in the medical area. This would limit real-life simulation of surface disinfectants produced for the veterinary and industrial areas |
| Efficacy testing product requirement | Only end products such as surface disinfectants and hand hygiene products are specified for efficacy testing | Efficacy testing are to be done to support efficacy of active ingredient and end product prior to product registration |
The main difference observed when compared to EN 14885 is that Phase 2, Step 1 tests are not required by NPRA for disinfectants used in healthcare institutions. Phase 2, Step 1 tests are suspension tests done to evaluate the efficacy of disinfectant liquid, whereas Phase 2, Step 2 tests are done to investigate the efficacy of the disinfectant—an evaluation that considers both the microbicidal activity and the application process of the disinfectant. Even though Phase 2, Step 2 tests are more difficult to pass compared to Phase 2, Step 1 tests, there are reasons why European Union Regulators have included both Phase 2 steps to claim product activity. It may be possible that the efficacy achieved in Phase 2, Step 2 tests are facilitated by the combined effects of the product and mechanical action. Thus, we think that Phase 2, Step 1 tests might still be valuable to include, in order to prove the activity achieved solely by the disinfectant liquid.
This blog was written solely based on our team’s interpretation of the NPRA guideline. TECOLAB is readily available to assist you in your surface disinfectant registration according to the NPRA guideline. We value your long-term investments as well by providing you with specific test strategy to accommodate your international registration needs. Let us know which country you would like to register your surface disinfectant today!

