OECD-GLP and ISO 17025 are two different types of ‘certificate’ that shows the competency of a laboratory to perform testing. Not all test methods performed in an ISO 17025 laboratory are accredited. In order to offer ISO 17025 accredited test methods, the laboratory needs to perform method validation or verification and calculate the measurement uncertainty of the test method. The laboratory is then audited by Standards Malaysia and if successful, the test method will be included in the laboratory’s accreditation scope.
On the contrary, an OECD-GLP laboratory is certified based on its area of expertise. For example, a laboratory that is GLP certified to perform toxicity studies indicates its capabilities to perform all the toxicology test methods under the GLP study. However, more resources and time is required to perform a GLP study compared to performing an ISO 17025 accredited test method. Hence, the test performed according to ISO 17025 is cheaper and quicker than one performed according to GLP.
Choose OECD-GLP certified test methods if your product requires toxicology studies that involve animal testing and ecotoxicological testing.
Laboratories that are accredited with ISO/IEC 17025 do have an added advantage when requesting for the compliance of OECD-GLP. The existing quality system implemented in an accredited laboratory can usually be adapted to OECD-GLP, and only additional requirements of the OECD-GLP regulation need to be fulfilled.
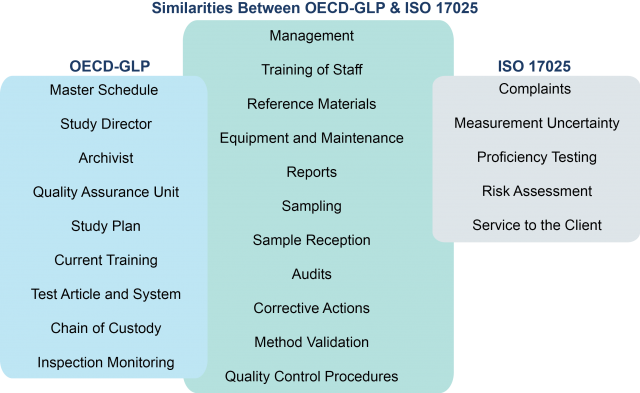
The figure above shows the similarities and differences between OECD GLP and ISO/IEC 17025.1
Should I choose OECD-GLP or ISO 17025 for my test?
Firstly, a disinfectant manufacturer should decide whether the product requires testing under ISO 17025, OECD-GLP, or neither. Here are some insights on the requirements:
Choose to perform test without ISO 17025 and or OECD-GLP certification if:
- If the test report is to be used internally only.
- The regulatory affairs of your country or the target country can accept test reports without ISO 17025 and/or OECD-GLP certification.
- There are no laboratories in the country that can perform the test under ISO 17025 and/or GLP.
Choose ISO/IEC 17025 accredited test methods if:
- The test method does not involve animal testing and/or environmental toxicology studies, and the regulatory affairs of the country can accept a test report without GLP certification.
- You want use the test report to support the claim of your products, including efficacy claims.
- It is intended for regular QC.
Choose OECD-GLP certified test methods if:
- Your product requires toxicology studies that involve animal testing and ecotoxicological testing, test data will be used for product registration purposes.
- The regulatory affairs require specific OECD test methods.
- You intend to use the test data in the SDS for product registration purposes, including chemical analysis.
If you need more assistance and information, do not worry. TECOLAB is able to provide efficacy tests to suit your product needs. Biocompatibility (animal toxicology) tests and ecotoxicological tests offered by TECOLAB can be performed under ISO 17025 accreditation or OECD-GLP certification based on your needs. Contact us to find out more.
Reference:
1. Engelhard, T., Feller, E., & Nizri, Z. (2003). A Comparison of the Complimentary and Different Issues in ISO/IEC 17025 and OECD GLP. Accreditation and Quality Assurance, 8(5), 208–212.

