“Hey, Lim! You know, with the whole pandemic situation going on, I have produced my own line of hand sanitizers to help combat the virus. By any chance, would you know how to sell my product in Malaysia?”—Have you manufactured a hand sanitizer product or are you assisting a foreign manufacturer to register their product in Malaysia? Well, this article’s just for you. In our previous blog, we dove into tips on claiming virucidal activity for hand sanitizers and today, we will be discussing on the steps for hand sanitizer registration and marketing strategies in Malaysia.
Before we jump into the different pathways of hand sanitizer registration, it’s important to know that other than surface disinfectants, all hand sanitizers in Malaysia are regulated by the National Pharmaceutical Regulatory Agency (NPRA). Hand sanitizers can be registered as a cosmetic product or as an over-the-counter (OTC) product (pharmaceutical product) depending on their usage, function, and active substance that narrows down to the specific product claim(s) you would like to market on your product label.
What is the Difference between a Cosmetic and Pharmaceutical Product?
According to NPRA, a cosmetic product would include any product topically exposed to human skin with an intention of changing the appearance, correcting body odor, protecting, and/or preserving its good condition. This could range from facial cream to hand sanitizers. Moreover, NPRA has also specifically defined skin antiseptics or disinfectants as non-scheduled poison (OTC) products or also referred to as low-risk pharmaceutical products—products containing safe active ingredients that are externally or topically applied and are used for non-critical conditions. Here are some brief differences to be consider when registering your product.
| DIFFERENCES | COSMETIC PRODUCT | PHARMACEUTICAL PRODUCT |
| Product Registration Duration | 1 month | 4 to 6 months, depending on the number of active ingredients |
| Product Claim | Limited to ‘antibacterial’ or ‘bactericidal activity’ only | More product claims can be made, e.g., antiviral, antifungal, mycobactericidal etc. |
| Laboratory Testing | Hand sanitizers with 60% ethanol do not require efficacy test report to support antibacterial claim.
Hand sanitizers with other active ingredients such as quaternary ammonium compounds (QACs) require efficacy test report to support antibacterial claim |
All hand sanitizer products require efficacy test to support product claims |
| License Requirement | No license required to sell product in Malaysia.’ Certificate of Free Sale’ is required if intended to sell product overseas | Either manufacturer, import, or wholesaler license is required to be applied according to the intended nature of registrant |
Registration Pathways
Cosmetic Product

Pharmaceutical Product
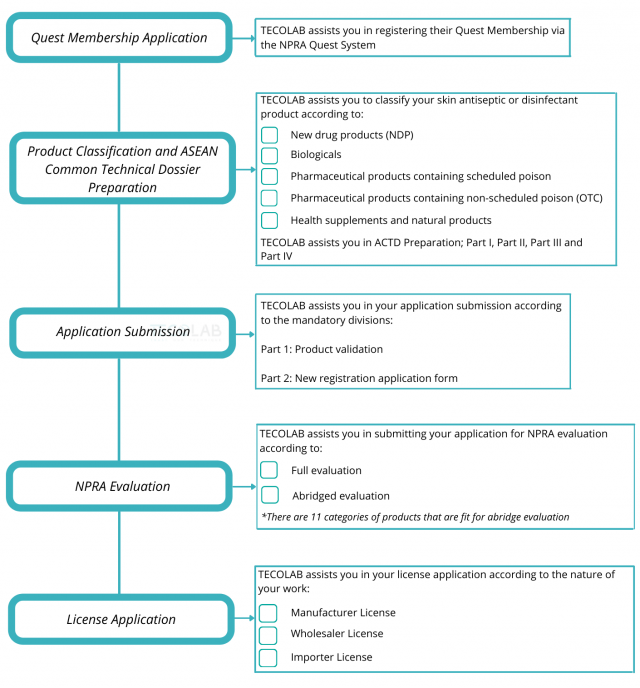
Which Pathway Should You Choose?
Have you decided which pathway you are interested in? Here are few things to consider when deciding a registration pathway for your product:
Product Claim and Efficacy Test Report
“I’ve registered my product as a cosmetic product, but with the current pandemic, I’d like to claim for virucidal activity. What should I do?”—As you’ve read above, cosmetic products are only limited to antibacterial claims. However, it is possible for a cosmetic product to upgrade to a pharmaceutical product. Product claims are supported by efficacy test report(s) that prove the effectiveness of these products against a specific or a group of microorganisms. If you have in mind the claims you would wish to achieve with your product, contact us further for a preliminary consultation during your product development to know more about our cost-effective efficacy test planned exclusively for your product knowing your product claim gives you a clearer insight in your product registration journey.
Costing and Time
“I don’t think we are able to wait too long to register our product.”—Cost and time are of utmost importance when developing or manufacturing a product. However, different manufacturers have different approaches to sell their products; some may prefer registering a product as a cosmetic product to analyze market behavior before investing for a pharmaceutical product. Others may only opt to sell a product as a cosmetic product whilst developing new products to register under the pharmaceutical pathway. Some may also choose to only sell locally. Let us know how we can assist you in working around your efficacy test budget and time to produce your money’s worth outcome for your product registration!
Product Marketing
“I wish I could claim more on my labels.”—Product marketing is essential to compete with the existing skin hygiene products that are in the current market. One of many strategies to increase product competitiveness is to have effectiveness against a broad microbial spectrum with a reasonable price. Registering a product in the pharmaceutical pathway allows you to claim more activities such as antifungal, antiviral, antituberculocidal, and more. These days, buyers tend to look into labels for a product that is effective against human coronavirus, influenza virus, and more. Having specific efficacy test reports supporting these specific claims, your pharmaceutical product may just be at the top of the game!
TECOLAB would like to advise you that the information in this blog are only recommendations and suggestions based on the interpretations of our team of experts. Let us know here your registration pathway of interest and if you’d like to know more on our cost-saving efficacy test plans!

