Assess the cytotoxicity of your medical devices, biocides, chemicals or cosmetics through in-vitro models.
Cytotoxicity testing is conducted using the MTT assay, a colorimetric method that measures cellular metabolic activity as an indicator of cell viability, proliferation, and cytotoxicity. This test is widely used to evaluate the in-vitro biocompatibility of medical device materials using cultured mammalian cells. The method can also be applied to assess the cytotoxic potential of biocides, chemicals, cosmetics, and other consumer products.
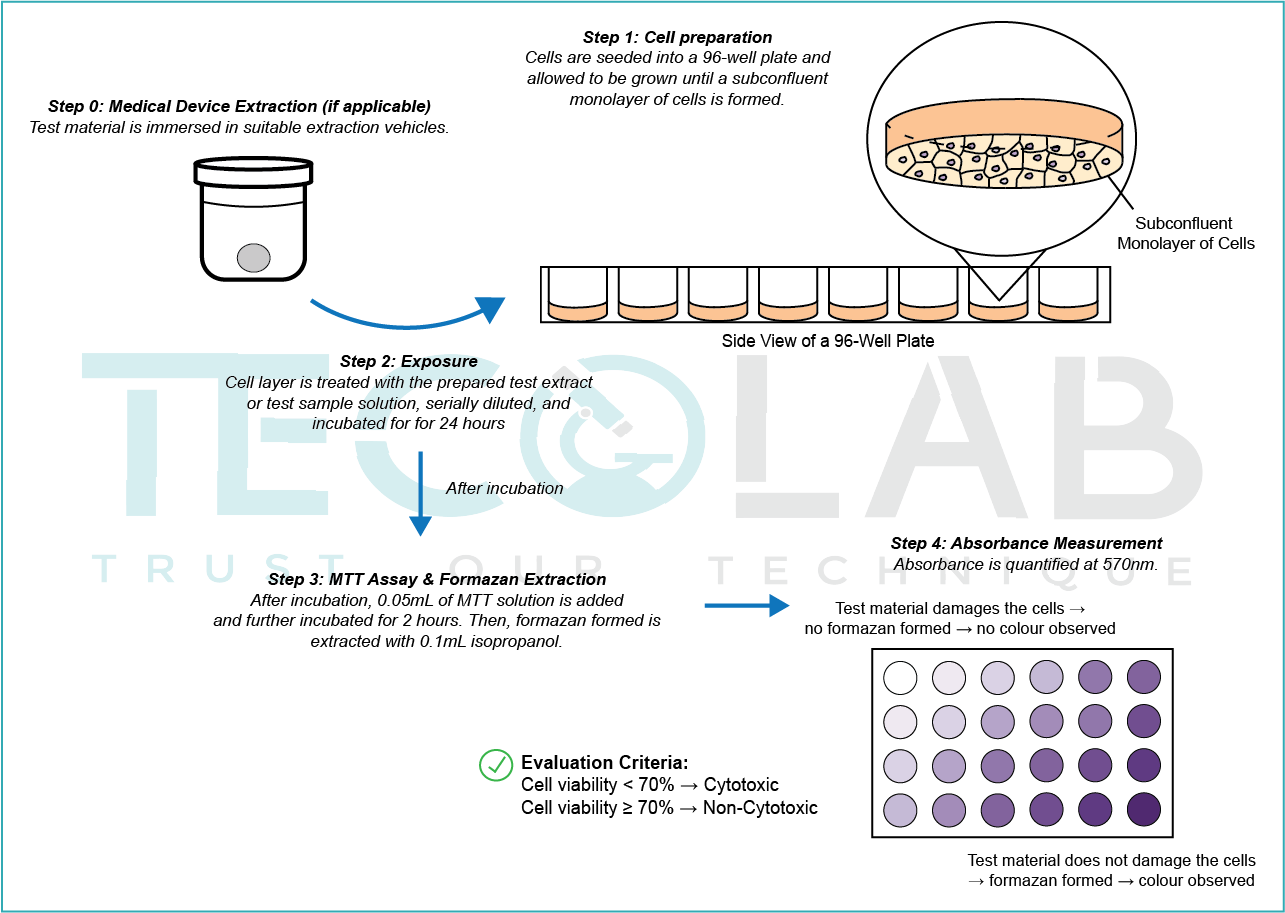
The diagram below provides a summary of the test method according to ISO 10993-5.
TESTING CRITERIA AND CUSTOMER PROCESS
Step 1: What is the sample preparation method?
- Cytotoxicity testing can be performed using either:
• Extracts of the test sample (most common for medical devices)
• Direct or indirect contact of the test material with the cell monolayer (used when extraction is not suitable)
Step 2: What is the suitable extraction time and condition?
Extraction must be carried out according to ISO 10993-12, with the typical conditions for standard extraction at 37 °C for 24 hours, extracted separately using both:
- Polar solvents (e.g., 0.9% sodium chloride, MEM with serum)
- Non-polar solvents (e.g., sesame oil or cottonseed oil)
For short-term contact devices (≤24 h exposure), extraction times may range from 4 to 24 hours, based on risk assessment.
Step 3: What are the exposure conditions?
A minimum of four concentrations of test sample/extract (including the undiluted extract) is applied to sub-confluent monolayers of a suitable cell line (commonly L929 mouse fibroblasts) and incubated for 24 hours.
Cell viability is calculated as a percentage relative to the untreated negative control.
Step 4: What is the passing criteria?
If cell viability is < 70%, the material is classified as cytotoxic.
If cell viability is ≥ 70%, the material is classified as non-cytotoxic.
Step 5: If the results show that the product is cytotoxic, what is the next step?
Further biocompatibility assessments can be performed using other in vitro assays, such as In vitro skin irritation tests (ISO 10993-23 or OECD 439)
A cytotoxic result does not always mean the product is unsafe; results should be interpreted in the context of clinical use, dose, exposure duration, and risk assessment.
Step 6: Place an order and send your sample to us
Fill in the test request form and place an order via email at info@tecolab-global.com. We will advise on the quantity of sample required for testing.
You can deliver the sample to us via courier or utilize our sample delivery service. We will monitor the delivery status and inform you when we receive the sample.
Step 7: Track the status of your request
Once we have received your sample, we will provide a tracking number to you. You can easily track the status of your request here.
Step 8: Download your password-protected test report
Download your readily available test report online with just a single click using the link and password provided via email notification. We will receive a notification once you have downloaded the test report.
For safety purposes, the uploaded test report will self-destruct after 7 days.
**Disclaimer: NO ANIMAL TESTING. TECOLAB is committed to ethical and cruelty-free testing practices. We do not conduct any form of testing on animals and all our biocompatibility tests are performed on in-vitro models.

