The bloom in the disinfectant market has conceivably led to the need to develop disinfectants or biocidal products displaying increasingly unique and enhanced antimicrobial claims to dominate the market. Through this, manufacturers seek out a variety of tests to substantiate more and more claims for their products which might be challenging yet rewarding and, in the end, completely worth it for the future of their products. Predicated solely on our experience and knowledge in this field, here, we list 5 of the most claim-worthy but challenging efficacy tests we offer by far.
5. EN 1500
 At number 5, we have the EN 1500 standard that evaluates the efficacy of hygienic handrub by assessing the reduction of bacteria on the hands after exposure. In this test, the handrub sample is applied onto the healthy hands of over 20 volunteers in order to simulate real-life conditions.
At number 5, we have the EN 1500 standard that evaluates the efficacy of hygienic handrub by assessing the reduction of bacteria on the hands after exposure. In this test, the handrub sample is applied onto the healthy hands of over 20 volunteers in order to simulate real-life conditions.
We find this standard fairly challenging due to the passing criteria for a product to be deemed suitable as a hygienic handrub. According to EN 1500, the test sample should demonstrate a mean reduction of bacteria that is comparable to that of the reference product, which is 60% propan-2-ol. Based on this, the efficacy of a variety of active substances including non-alcohol-based formulations or with natural ingredients must be equivalent or superior to that of alcohol which we have witnessed, on occasion, to be tough. Besides that, sufficient product to cover the average hand size throughout the application procedure and contact time also plays a vital role which might be overlooked.
4. Full Virucidal Activity in EN 14476
EN 14476 is a suspension test, which methodology wise, should be pretty simple, isn’t it? Why is it challenging? Yes, it is and yes, suspension tests in general are overall simpler. But the type of test is not what makes this claim difficult to achieve—it is because of the properties of the obligatory non-enveloped virus strains to obtain the claim of full virucidal activity, specifically poliovirus. Let us dive into this.
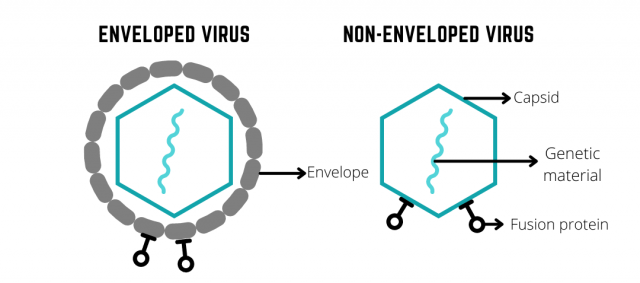
Viruses are classified into enveloped and non-enveloped virus based on their structure. In the presence of chemical disinfectants, the envelope of enveloped viruses is much easier to be disintegrated as compared to the capsid layer of non-enveloped viruses, therefore making non-enveloped viruses more resistant to disinfectants. More than that, non-enveloped viruses are further categorized based on size—large and small non-enveloped virus—and small non-enveloped viruses such as poliovirus have been found to be highly resistant to biocides. For more in-depth information on the differences between enveloped and non-enveloped viruses, check out our previous blog post.
Alcohol such as ethanol and isopropyl alcohol is widely used in the formulation of a variety of products from hand sanitizers to surface disinfectants. This is also the recommended active substance for handrubs by international agencies such as CDC and WHO. While formulations containing 60% to 80% of alcohol is able to effectively kill most microorganisms from bacteria, yeast and enveloped viruses, there are exceptions to the rule. Formulations with up to 80% have been found to unsuccessfully demonstrate complete inactivation of enteroviruses such as poliovirus after 5 minutes of contact time (Kampf, 2018).
That being said, it is not impossible to achieve full virucidal activity for products. For example, in a study (Steinmann, 2010), formulations containing alcohol mixed with acid has shown to demonstrate better efficacy against poliovirus.
3. Sporicidal Activity (EN 17126)
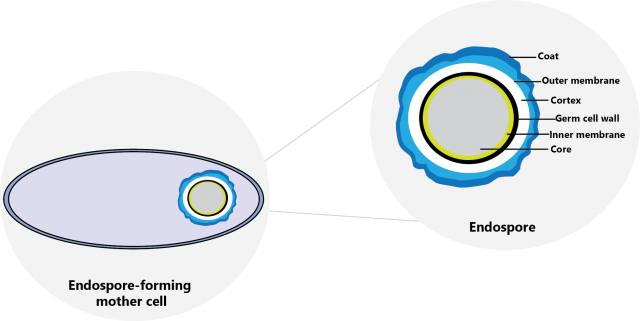
On the same idea of specific microorganisms showing varying levels of resistance towards biocides, bacterial spores are one of the most resistant microorganisms there is. Some common examples of bacterial spores are Bacillus cereus, Bacillus subtilis and Clostridium difficile. What makes spores so challenging to kill is their cellular structure consisting of an outer spore coat which prevents them from dying in unfavourable conditions, including in the presence of chemical disinfectants. Although difficult, there are certain active substances such as peracetic acid and hydrogen peroxide that have shown high efficacy against spores (CDC, 2008).
2. EN 17272 – Automated Airborne Disinfection System
 Airborne disinfectant devices are swarming the market as such a device works wonders in simplifying the task of complete room disinfection whether for use in medical area, industrial area such as manufacturing sites, domestic area, and others. In 2020, a standard method EN 17272 was published to not only evaluate the efficacy of the disinfectant released by the automated machine but also how well the machine is able to distribute the disinfectant throughout the enclosure effectively.
Airborne disinfectant devices are swarming the market as such a device works wonders in simplifying the task of complete room disinfection whether for use in medical area, industrial area such as manufacturing sites, domestic area, and others. In 2020, a standard method EN 17272 was published to not only evaluate the efficacy of the disinfectant released by the automated machine but also how well the machine is able to distribute the disinfectant throughout the enclosure effectively.
Why is this test method so challenging, you ask? Well, there are several minor details that we have encountered that may have been overlooked when formulating and developing the product which can easily cause the product to be unsuccessful in its goal. For one, the amount of product being dispersed throughout the room plays a major role. Full coverage of the surfaces with the product is crucial to allow for antimicrobial activity to take place effectively. Not only that, the product should also be able to remain on the surface and not drip, fall, or evaporate off throughout the chosen disinfection contact time. The distribution of the product is important as well, ensuring that the machine or generator is capable of distributing the disinfectant to all corners of the enclosure.
All in all, it is once again not impossible to have an effective airborne disinfectant machine so long as the little but important details are not overlooked to ensure its efficacy.
1. PAS 2424
The number 1 most challenging efficacy test is PAS 2424. One of the most popular claims seen in the market is for residual antimicrobial activity such as “24-hour protection against bacteria”. This is where the PAS 2424 standard is developed and published to recreate real-life conditions under a laboratory setting in order to evaluate the residual activity of disinfectant products applied on hard surfaces.
What makes the PAS 2424 much more challenging is the introduction of multiple wet and dry abrasion cycles onto the surface after application of the product followed by reinoculation of microorganisms in efforts to simulate how a general surface will be treated day in and day out. As we know, the presence of sufficient product that comes in contact with the microorganism is key for it to perform effectively. However, the product available on the surface is at risk of being removed after each abrasion, making it more challenging to demonstrate its antimicrobial efficacy against the newly inoculated microorganism. Despite being one of the most challenging tests we have encountered, passing this test could do wonders for the product claim and marketability which would be truly rewarding.
What now?
Don’t feel discouraged to perform these tests and achieve the claims for your products! Our advice is just to be mindful of the claims you would like and aim for it. We at TECOLAB understand that product testing can be costly and we hope to help you through the R&D process of your products before diving into performing the full test. Let us know the goal for your product claims and we are happy to help you achieve it.
TECOLAB would like to declare that the information in this blog is solely based on the interpretation, knowledge, and experience of our team of experts in the field of efficacy testing with no specific product information in mind.
References:
1. Steinmann, J., Becker, B., Bischoff, B., Paulmann, D., Friesland, M., Pietschmann, T., … & Steinmann, E. (2010). Virucidal activity of 2 alcohol-based formulations proposed as hand rubs by the World Health Organization. American journal of infection control, 38(1), 66-68.
2. Kampf, G. (2018). Efficacy of ethanol against viruses in hand disinfection. Journal of Hospital Infection, 98(4), 331-338.
3. Hand sanitizer use out and about. (2021). Centers for Disease Control and Prevention (CDC). https://www.cdc.gov/handwashing/hand-sanitizer-use.html
4. WHO Guidelines on Hand Hygiene in Health Care. First Global Patient Safety Challenge: Clean Care is Safer Care. (2009). World Health Organization (WHO). https://www.who.int/publications/i/item/9789241597906
5. Guideline for Disinfection and Sterilization in Healthcare Facilities. (2008). Centers for Disease Control and Prevention (CDC). https://www.cdc.gov/infectioncontrol/guidelines/disinfection/disinfection-methods/chemical.html

