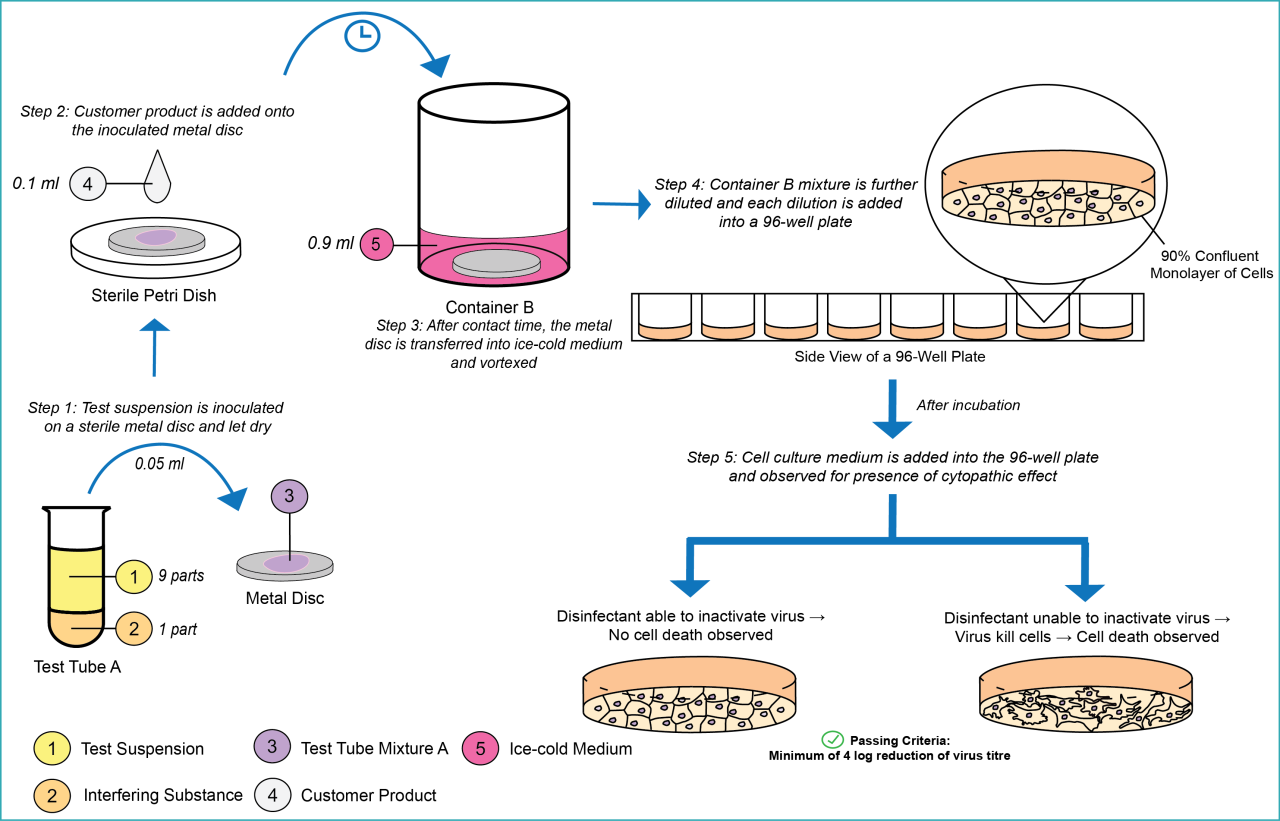
This test is suitable to evaluate surface disinfectants that require no wiping action used in the medical area.
These are the mandatory virus strains for testing according to virucidal activity claims:
Virucidal Activity:
Δ The product shall pass against Poliovirus, Adenovirus and Murine Norovirus in EN 14476 prior to EN 16777
Limited Spectrum Virucidal Activity:
Δ The product shall pass against Adenovirus and Murine Norovirus in EN 14476 prior to EN 16777
Virucidal Activity Against Enveloped Virus:
Δ The product shall pass against Vaccinia virus in EN 14476 prior to EN 16777
Wish to add more microorganisms? Click here for our full list of microorganisms.
The test concentration is chosen according to the manufacturer’s recommendation.
The contact time recommended by the test method is no longer than 5 minutes for surfaces frequently touched by different people, and no longer than 60 minutes for other surfaces.
Interfering substances, or commonly referred to as soiling, are used to mimic organic substances found on human skin, surfaces, and instruments. These are the available soiling conditions according to the test method:
Fill in the test request form and place an order via email at info@tecolab-global.com. We will advise on the quantity of sample required for testing.
You can deliver the sample to us via courier and provide us the tracking code. We will monitor the delivery status and inform you when we receive the sample.
Once we have received your sample, we will provide a tracking number to you. You can easily track the status of your request here.
Download your readily available test report online with just a single click using the link and password provided via email notification. We will receive a notification once you have downloaded the test report.
For safety purposes, the uploaded test report will self-destruct after 7 days.
In order to pass the test, the product shall demonstrate at least a 4 decimal log (lg) reduction.
Need assistance with your test report? Discuss your results with our microbiologists for more information.