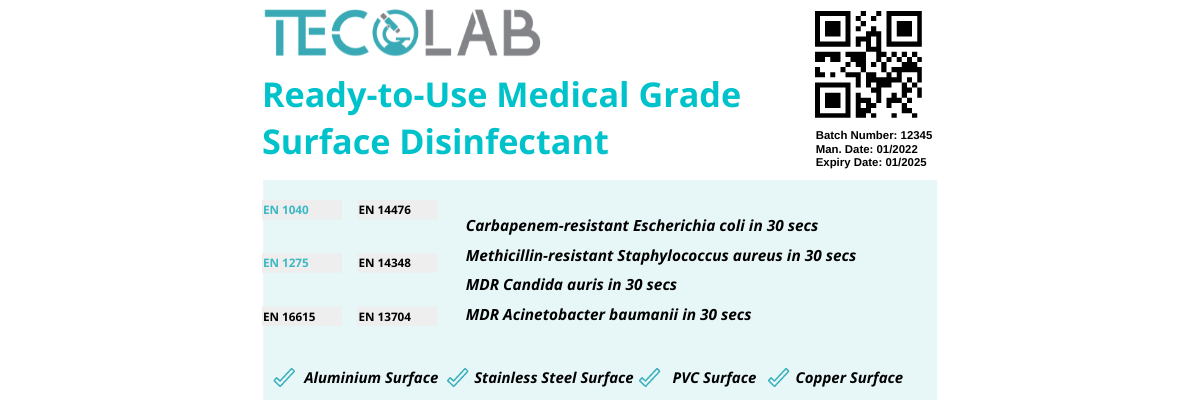
“Hey, Tim. Could you remind me of what I had to check for on the label? I’m looking at the bottle right now.”—You and I can both agree that our label reading tendencies has, at the drop of a hat, increased amongst hand sanitizers, surface disinfectants, and even towards face mask due to the current pandemic. Just as how we scout for golden words or phrases like “paraben-free” on shampoo bottles, or even “potato chunks” on a can of soup, disinfectants have their own “Extra 20% Filling” you should look out for. This blog describes the tips on reading disinfectant labels and the minimum information required on a disinfectant label according to EN 14885:2018. Take a look at the example of a surface disinfectant product label with a few mislabels. Let’s break them down together in this blog.

Where are the Common Mislabels?
EN 14885:2018, as we know, is the godfather of disinfectant efficacy testing norms according to European Standard and it describes the minimum information required for the common categories of disinfectants found in our current market. The recommended information to be included are:
- The type and/or purpose of product
- The area and field of product application
- The antimicrobial spectrum of product
The common mislabels usually happen with the information that correspond to these recommended information. This can be observed in the direction of use, product efficacy claims, contact time, and tested microorganism. Not sure where to start on the bottle? Check out the few steps below!
Step 1: Identify the Product Application, Type, and Grade of Disinfectant

“Hmmm, it says ‘medical grade’ on the bottle. Is there a specific reason why?”—The most important element to consider when purchasing a disinfectant product, would be the product application. As you know, a variety of disinfectants exist in the current market. The intended use of the product often varies amongst disinfecting skin and/or hands, surfaces of facilities or medical devices, surgical or dental instruments. According to the label above, it says this product is a ready-to-use, medical grade, hard surface disinfectant. This sentence identifies the type and/or purpose of the disinfectant: a disinfectant used on hard surfaces (the field of application) in the medical area (the area of application). It is also important to know that these area claims should be supported with the appropriate efficacy tests that takes into consideration the appropriate conditions such as soiling and surrounding temperature of the targeted area. Our blog here describes more on the varying conditions in different targeted areas.
Step 2: Identify the Suitability and Practicality of Disinfectant
Active Ingredient and Material Compatibility of Disinfectant

“This product seems really promising, but is it suitable to disinfectant my table?”—Just as how a cold drink without a coaster leaves a permanent water ring mark on our wooden tables, exposing sensitive surfaces to chemicals may cause similar effects. It is important to take note of the active ingredient and material compatibility of the product towards the surfaces you’d wish to expose it on. For example, based on the mock label above, this product has great suitability towards aluminum, stainless steel, PVC, and even copper surfaces. Products as such have been subjected to material compatibility assessments. The active substance of the product—whether it is non-alcohol or alcohol-based— does play a big role towards product compatibility. Certain active substance may cause corrosion, discoloration, or cracking towards different type of materials. Check out our blog here that summarizes the pros and cons of the common active substances, including material compatibility, used in disinfectant production.
Antimicrobial Spectrum and Efficacy Claims of Disinfectant

”Hey, look! It says fungicidal activity with Candida albicans. Is this appropriate for the mold in my house?” —Apart from contributing to the material compatibility of the product, the type and concentration of active substance in a disinfectant is usually also a hot potato amongst disinfectant manufacturers. This is because the formulation of the disinfectant—whether a combination of active substance—contributes to the antimicrobial spectrum of the disinfectant. These antimicrobial spectrum can be predicted by performing appropriate efficacy testing. As seen on the label above, this product has multiple efficacy testing done to support its antimicrobial claims. As you know by now from our blog here, Phase 1, Step 1 efficacy tests such as EN 1040 and EN 1275 are not suitable for product claims as compared to Phase 2, Step 1 and Phase 2, Step 2 tests. This is because Phase 1, Step 1 tests do not take into consideration the common soiling (interfering substance) during product application. These common soiling can represent dust on hands, blood residue on instruments, urine residue on patient skin, and more. One of the claims of our mock disinfectant had also included fungicidal activity with Candida albicans. Sadly the answer to our bot question would be no, it will not be effective against the mold in his house. This is because, in order to claim fungicidal activity, Phase 2, Step 1 and Phase 2, Step 2 efficacy testing must be done against both Aspergillus brasiliensis and Candida albicans.
Apart from test microorganism, end users and disinfectant manufacturers should note that the kill time corresponding to product application is also important. One of our top advices to disinfectant manufacturers in regards to choosing a suitable contact time would be identifying the practicality of their product. As noticed on the disinfectant label above, it shows mycobactericidal activity in 15 minutes and sporicidal activity in 20 minutes. Although having passed the efficacy tests, the kill time or contact time is not practical for real-life application. This is because within the 15 minutes and 20 minutes, the disinfectant may have already evaporated and produces a slower kill reaction. This is not ideal for infection-prone areas such as the healthcare settings. Moreover, it would also be tough to avoid consumer or patient contact onto the treated surface for 15-20 minutes in a crowded and fast-paced environment. Our lab offers affordable screening packages that allows you to determine the best contact time for your product. Click here to learn more on how contact time contributes to product uniqueness.
Step 3: Safety Precautions of Disinfectant

“Hey, Spence. It says here that gloves are to be worn when handling product during application. Let me get you a pair.”—Safety precautions are a must-read before using a product. Chemical disinfectants and antiseptics often pose a risk to humans via the exposure to direct skin contact, eyes, inhalation, and/or even accidental ingestion. The safety towards human health and storage conditions of disinfectants are mandatory evaluation requirements prior to product registration and market placement. Personal Protective Equipment (PPE) such as gloves, mask, and eye protection are recommended to be worn when handling high-risk chemical disinfectants. Some disinfectants have special storage conditions such as “avoid direct heat or sunlight” due to being flammable or reactive in high temperature that may affect the efficacy of the product. The storage condition of products may also vary according to the active substance of the product. For example, hydrogen peroxide-based disinfectants are to be kept away from direct heat due to the increase in active substance degradation in the presence of high heat.
Do you see or have a product claim that you are unsure of? Call us today to speak to one of our experts to know more. Get bonus expertise advice on the suitable product claims, application, and affordable efficacy tests to support your disinfectant product.