Surgical Hand Disinfection
EN 12791 is an essential European Standard used to simulate practical conditions for establishing whether a surgical handrub or handwash reduces the release of resident and eventually present transient microbial flora on hands. The infographic below shows a brief insight of EN 12791.
Learn more about our customer process to book a test.
CUSTOMER PROCESS
Step 1: Is your product a surgical handrub or handwash?
Step 2: What is the test organism and number of volunteers required?
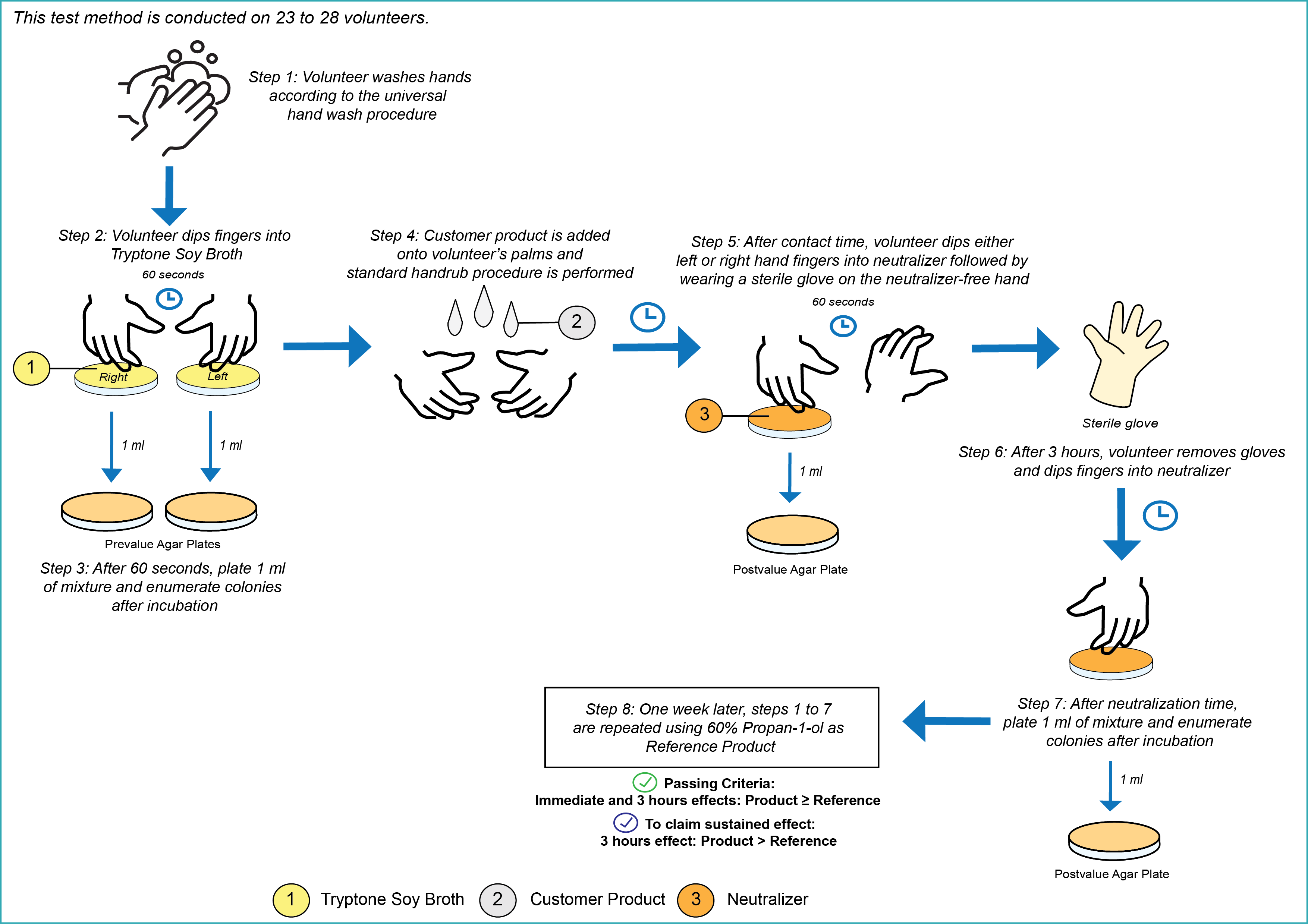
EN 12791 uses the transient microbial flora found on the skin of human hands.
The number of volunteers required is 23 to 28.
Step 3: Select an appropriate contact time and application procedure for your test product
The contact time is according to the manufacturer’s recommendation, but not shorter than 60 seconds and not longer than 5 minutes.
Please also provide us with the application procedure, which shall include the volume of product and the frequency of application.
Step 4: Place an order and send your sample to us
Fill in the test request form and place an order via email at info@tecolab-global.com. We will advise on the quantity of sample required for testing.
You can deliver the sample to us via courier and provide us the tracking code. We will monitor the delivery status and inform you when we receive the sample.
Step 5: Track the status of your request
Once we have received your sample, we will provide a tracking number to you. You can easily track the status of your request here.
Step 6: Download your password-protected test report
Download your readily available test report online with just a single click using the link and password provided via email notification. We will receive a notification once you have downloaded the test report.
For safety purposes, the uploaded test report will self-destruct after 7 days.
Step 7: Analyze your passing criteria in the test report with our laboratory experts
In order to pass the test, the product shall be at least not inferior to the reference product (60% Propan-1-ol). To additionally claim a sustained effect, the 3-hour effect of the product shall be superior to that of the reference product.
Need assistance with your test report? Discuss your results with our microbiologists for more information.

