Have you seen that NPRA released the new Guidelines for Control of Cosmetic Products in Malaysia? This now includes more informative guidelines for registering hand sanitizer and hand wash products, especially so for non-alcohol-based hand sanitizer. Before we dive deeper into the new requirements for registering your hand sanitizers with NPRA, are you aware that there are two categories for registering hand sanitizers, as a cosmetic product or as an over-the-counter (OTC) product (pharmaceutical product)? Let us first discover which your product falls under.
How do you know if your product classifies as cosmetic product?
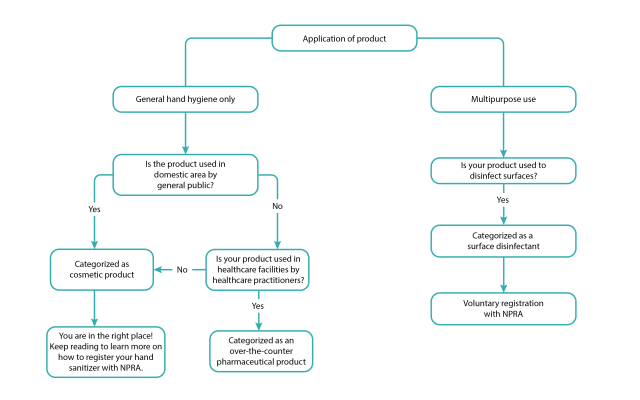
Now that we know the category of your hand sanitizer and/or hand wash, take a look at a summary of the differences between a cosmetic product and pharmaceutical product registration.
| DIFFERENCES | COSMETIC PRODUCT | PHARMACEUTICAL PRODUCT |
| Product Registration Duration | Around 2 weeks | 4 to 6 months |
| Product Claim | ‘Antibacterial’ claim only | Various product claims allowed, e.g., antiviral, antifungal, etc. |
Feel free to visit this blog post for a more detailed explanation and our expert tips on deciding on the best pathway for your product.
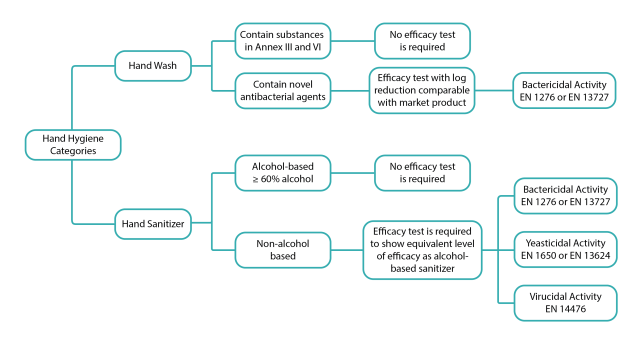
Do you need efficacy testing in order to register your hand sanitizer?
Having cosmetic products registered through a notification process with NRPA has made this process a lot quicker and simpler for cosmetic products to be approved and ready to be marketed. However, this is only true for alcohol-based products, namely hand sanitizers that contain at least 60% alcohol as the active substance and hand wash products containing substances listed in Annex III and Annex VI. On the other hand, non-alcohol-based products, namely hand sanitizers with any other active substances such as quaternary ammonium compounds, hypochlorous acid, etc., requires more documentation and efficacy evidence in the notification process. Let’s take a look at the different efficacy requirements for different categories.
How can TECOLAB assist you?
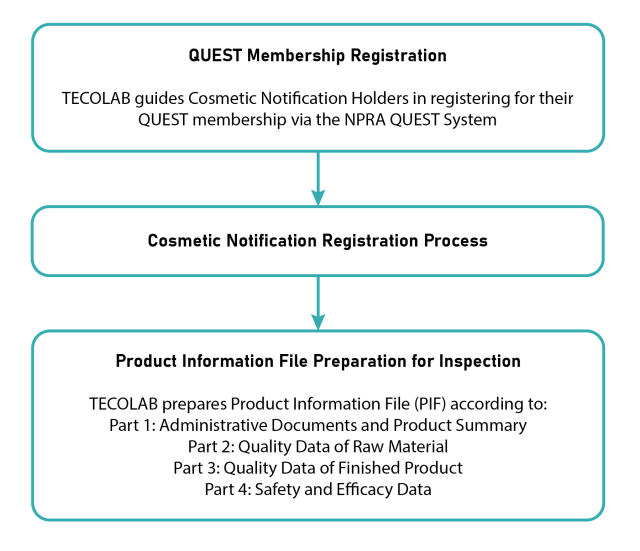
This blog was written solely based on our team’s interpretation of the NPRA guideline and communication with NPRA. Our experts here at TECOLAB have assisted in the process of several hand sanitizers hitting the market either locally manufactured or internationally imported. We will advise you on all the necessary testing, review your documentation and register your hand sanitizer for you. Let us know here about the plans for your product and we will consult you accordingly to meet all your product registration needs.
References:
- NPRA – Guidelines for Control of Cosmetic Products in Malaysia Annex I, Part 9 (ii)
- NPRA – Guidelines for Control of Cosmetic Products in Malaysia Annex I, Part 9 (iii)

